Molybdenum »
PDB 4c80-5koj »
5chc »
Molybdenum in PDB 5chc: Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps
Protein crystallography data
The structure of Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps, PDB code: 5chc
was solved by
C.-L.Tsai,
J.A.Tainer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.52 / 2.38 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 133.926, 176.022, 193.691, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17 / 22.7 |
Other elements in 5chc:
The structure of Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps also contains other interesting chemical elements:
| Zinc | (Zn) | 3 atoms |
| Iron | (Fe) | 57 atoms |
| Sodium | (Na) | 8 atoms |
Molybdenum Binding Sites:
The binding sites of Molybdenum atom in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps
(pdb code 5chc). This binding sites where shown within
5.0 Angstroms radius around Molybdenum atom.
In total 3 binding sites of Molybdenum where determined in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps, PDB code: 5chc:
Jump to Molybdenum binding site number: 1; 2; 3;
In total 3 binding sites of Molybdenum where determined in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps, PDB code: 5chc:
Jump to Molybdenum binding site number: 1; 2; 3;
Molybdenum binding site 1 out of 3 in 5chc
Go back to
Molybdenum binding site 1 out
of 3 in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps
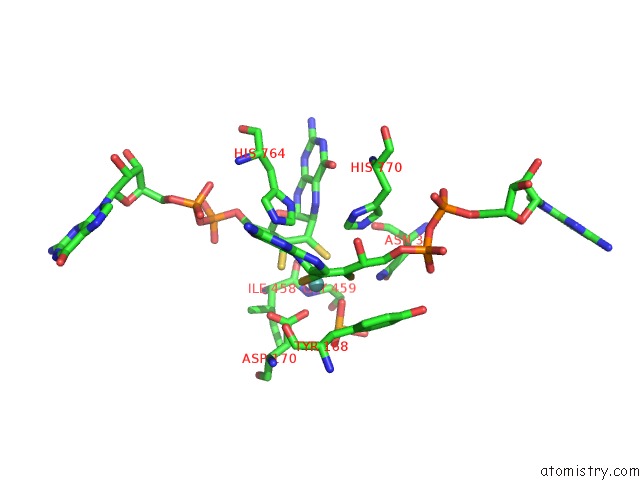
Mono view
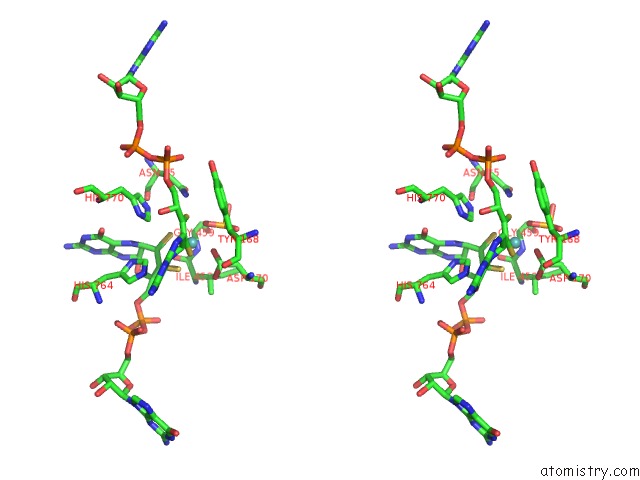
Stereo pair view

Mono view

Stereo pair view
A full contact list of Molybdenum with other atoms in the Mo binding
site number 1 of Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps within 5.0Å range:
|
Molybdenum binding site 2 out of 3 in 5chc
Go back to
Molybdenum binding site 2 out
of 3 in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps
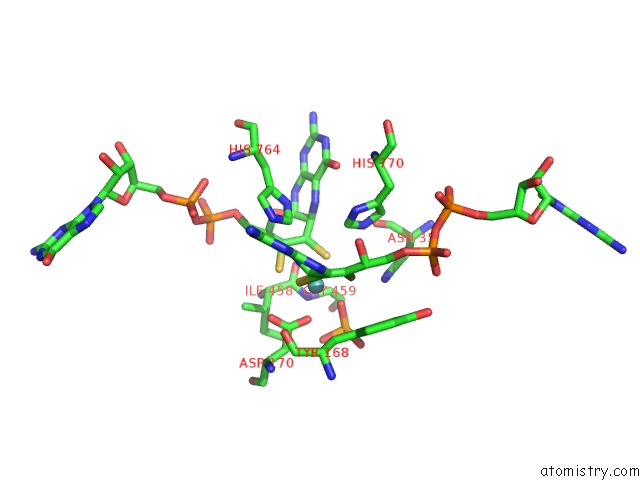
Mono view
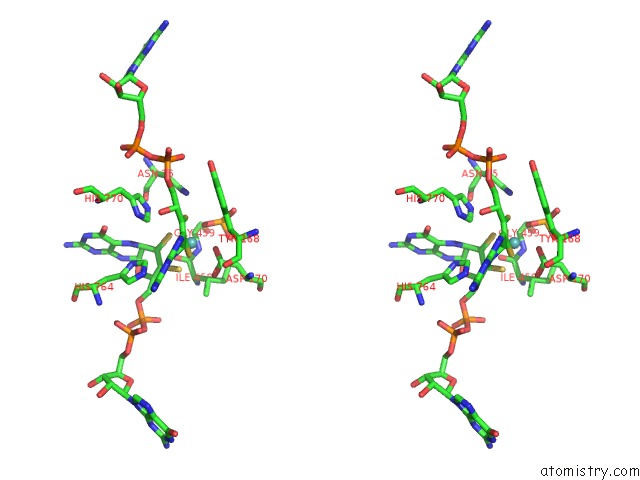
Stereo pair view

Mono view

Stereo pair view
A full contact list of Molybdenum with other atoms in the Mo binding
site number 2 of Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps within 5.0Å range:
|
Molybdenum binding site 3 out of 3 in 5chc
Go back to
Molybdenum binding site 3 out
of 3 in the Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps
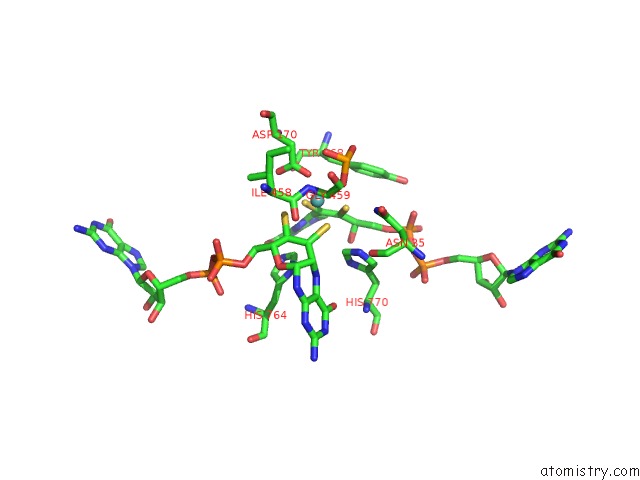
Mono view
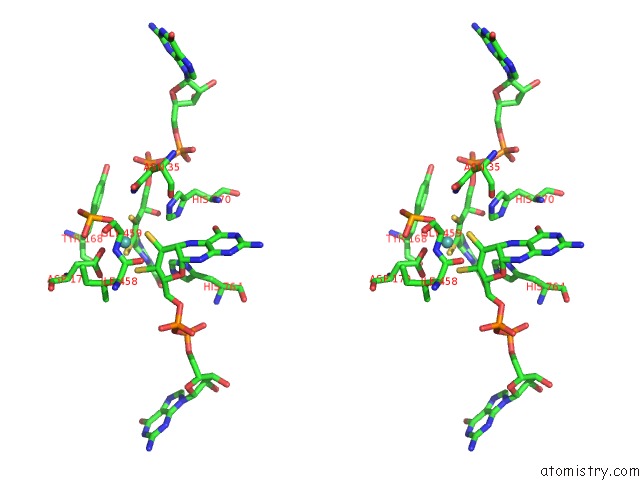
Stereo pair view

Mono view

Stereo pair view
A full contact list of Molybdenum with other atoms in the Mo binding
site number 3 of Crystal Structure of the Perchlorate Reductase Pcrab - Substrate Analog SEO3 Bound - From Azospira Suillum Ps within 5.0Å range:
|
Reference:
M.D.Youngblut,
C.L.Tsai,
I.C.Clark,
H.K.Carlson,
A.P.Maglaqui,
P.S.Gau-Pan,
S.A.Redford,
A.Wong,
J.A.Tainer,
J.D.Coates.
Perchlorate Reductase Is Distinguished By Active Site Aromatic Gate Residues. J.Biol.Chem. V. 291 9190 2016.
ISSN: ESSN 1083-351X
PubMed: 26940877
DOI: 10.1074/JBC.M116.714618
Page generated: Sun Oct 6 16:20:13 2024
ISSN: ESSN 1083-351X
PubMed: 26940877
DOI: 10.1074/JBC.M116.714618
Last articles
Ca in 3KM5Ca in 3KLT
Ca in 3KL6
Ca in 3KLL
Ca in 3KHR
Ca in 3KLK
Ca in 3KHL
Ca in 3KHE
Ca in 3KKF
Ca in 3KHH